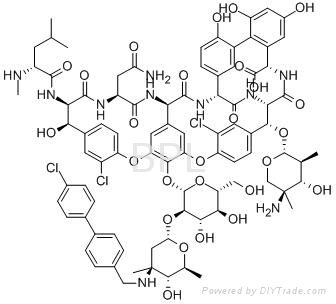
Oritavancin / 171099-57-3
Product Description
Oritavancin (LY333328/Orbactiv) is a novel semisynthetic glycopeptide antibiotic being developed for the treatment of serious Gram-positive bacterial infections. In December 2008, the US FDA declined to approve it without additional studies, and an EU application was withdrawn. In 2009, the development rights were acquired by The Medicines Company, which has completed clinical trials and submitted a new drug application to the FDA in February 2014. On August 6, 2014, the FDA approved oritavancin for treatment of skin infections in the United States. Its chemical structure is similar to vancomycin. It is a lipoglycopeptide.
| Payment Terms: | TT/Western Union |
| |
Product Image
Img 1
Send Inquiry to this Member
Related Products of this Company
This member assumes full responsibility for the content of this listing. DIYTrade accepts no responsibility whatsoever in respect of such content.
To report fraudulent or illegal content, please
click here.
China Suppliers Quick Searching:
,