Tantalum stents made of tantalum wire
Product Description
|
Material : Tantalum stent made of tantalum wires
Temper : annealed
Specs : according to ASTM F 560 -98 R05400 or ISO 5832-3
Chemical composition :
O 0.0300 %max
C 0.0100 %max
N 0.0100 %max
H 0.0015 %max
Fe 0.0100 %max
Ni 0.0100 %max
Si 0.0050 %max
Mo 0.0200 %max
W 0.0500 %max
Nb 0.1000 %max
Ta balance (typically 99.95%)
Physical & Mechanical property :
Tensile strength 172 MPa min.
Yield strength 103 MPa min.
Elongation 25%min
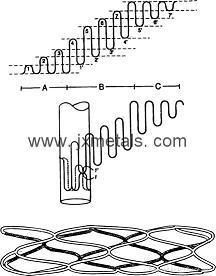
Size : made of tantalum filament with 0.15mm up to 0.50mm diameter Straight tantalum wire can be fabricated into Tantalum stent, straight tantalum wire i s formed into a wave pattern. Wrapping of the formed wire to line up U points, which are then welded. Finished device is tantalum expanded mesh, similar to polyetherurethane-coated tantalum wire stents used in the experiment.
|
Typical Sizes :
Ø 0.15mm(0.007") x coil;
Ø 0.20mm(0.007") x coil;
Ø 0.245mm(0.007") x coil;
Ø 0.25 mm(0.010") x coil;
Ø 0.30 mm(0.012") x coil;
Ø 0.38 mm (0.015")x coil;
Ø 0.50 mm x coil ;
Application:
Radiopaque tantalum for medical devices:
A radiopaque tantalum medical device such as a stent for use with or implantation in a body lumen is disclosed. The stent is made from a superelastic alloy such as tantalum, nickel-titanium or nitinol, and includes a ternary element selected from the group of chemical elements consisting of iridium, platinum, gold, rhenium, tungsten, palladium, rhodium, tantalum, silver, ruthenium, or hafnium. The added ternary element improves the radiopacity of the tantalum stent comparable to that of a stainless steel stent of the same size and strut pattern coated with a thin layer of gold. The tantalum stent has improved radiopacity yet retains its superelastic and shape memory behavior and further maintains a thin strut/wall thickness for high flexibility.
Standard Specification for Unalloyed Tantalum for Surgical Implant Applications (ASTM F 560 - 05 UNS R05200, ASTM F 560 - 05 UNS R05400). This specification is under the jurisdiction of ASTM committee F04 on Medical and Surgical Materials and Devices (orthopaedic medical devices) and is the direct responsibility of Subcommittee F04.12 on Metallurgical Materials.This Specification covers the chemical requirements, mechanical properties, and metallurgical requirements for unalloyed tantalum wire, unalloyed tantalum rod, unalloyed tantalum tube, unalloyed tantalum ribbon, unalloyed tantalum strip, unalloyed tantalum foil, unalloyed tantalum sheet, unalloyed tantalum plate, etc., used in the manufacture of surgical implants.Referenced Standards :
ASTM B 364 Specification for Tantalum and Tantalum Alloy Ingots;
ASTM B 365 Specification for Tantalum rod + Tantalum Alloy Rod + Tantalum Wire +Tantalum Alloy Wire;
ASTM B 708 Specification for Tantalum Ribbon, Tantalum Alloy Ribbon, Tantalum Strip, Tantalum Alloy Strip, Tantalum Foil, Tantalum Alloy Foil, Tantalum Sheet, Tantalum Alloy Sheet, Tantalum Plate, Tantalum Alloy Plate;
ASTM E 8 Test Methods for Tension Testing of Metallic Materials;
ASTM E 29 Practice for Using Significant Digits in Test Data to Determine Conformance with Specifications;
ASTM F 981 Practice for Assessment of Compatibility of Biomaterials for Surgical Implants with Respect to Effect of Materials on Muscle and Bone;
ASQ C1 Specification of General Requirements for a Quality Program;
ISO 6892 Metallic Materials Tensile Testing at Ambient Temperature;
Product Image
Img 1

Img 2
Send Inquiry to this Member
Related Products of this Company
This member assumes full responsibility for the content of this listing. DIYTrade accepts no responsibility whatsoever in respect of such content.
To report fraudulent or illegal content, please
click here.
China Suppliers Quick Searching:
,