| Model: | BK-0001 |
|---|---|
| Brand: | BIOCUP |
| Origin: | Made In China |
| Category: | Home Supplies / Personal Care Appliance |
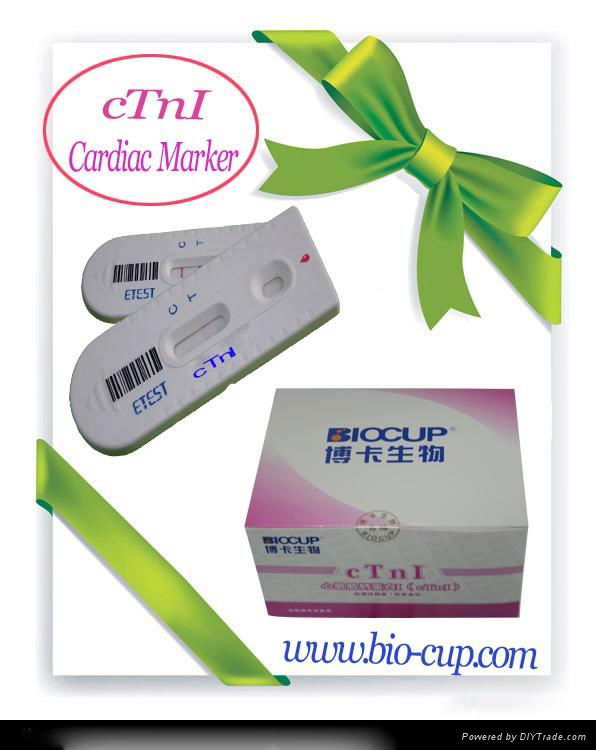
| Label: | cTnI , Cardiac Marker , POCT |
| Price: |
US $4
/ Pc
|
| Min. Order: | 100 Pc |
Product Description
 iTest Cardiac Marker
iTest Cardiac Marker
Immune and Quantitative Analysis System
The Platform of Rapid Test of Cardiac Marker POCT
GR200 Immune and Quantitative Analyzer + Cardiac Marker Reagent
GR200 Immune and Quantitative Analysis System
GR200 Immune and Quantitative Analyzer

○ The principleof Test
The instrument analyzes the result through the photoelectric sensing technology,obtain a final,quantitative result for test.
○The Characteristics of Instrument
The function of selection for sample type,solving the difference for sample,the result is more accurate.
With two kinds of modes for test,enabling to realize batch for test.
Storage space of large capacity,enabling the result of test for large quantity.
A platform is application to a variety of test items,fit the the need of clinical rapid diagnosis.
The Test of Myocardial Infarction and Damage
Physical and Chemical Characteristics of Myocardial Three Items
|
Troponin I ( cTnI ) |
Myoglobin ( Myo ) |
Creatine Kinase ( CK-MB ) |
|
4-8 hours,rising
|
1-3 hours,rising
|
3-8 hours,rising
|
|
The Gold Standard of Test of Myocardial Damage |
The Important Index of Exclusion of AMI Early Negative / The Most Sensitive Index of Recrudescent Test for AMI |
The Marker of Non-Q-wave Myocardial Infarction / The Ideal Marker of Interventional Therapyfor Myocardial Damage |
○ The Diagnostic Significance of Three Markers
|
The Result of Test |
Signifiicance |
||
|
cTnI |
CK-MB |
Myo |
|
|
+ |
+ |
+ |
AMI occurs within 12 hour. |
|
+ |
+ |
- |
AMI occurs more than 12 hours. |
|
+ |
- |
+ |
Basicly,can make sure to occur AMI. |
|
+ |
- |
- |
AMIoccurs in 24-96 hours. |
|
- |
+ |
+ |
Muscle or myocardial damage in the early stage,recommended to test continuously within 4-8 hours. |
|
- |
+ |
- |
Muscle or myocardial damage in the early stage,recommended to test continuously within 4-8 hours. |
|
- |
- |
+ |
Muscle or myocardial damage in the early stage,recommended to test continuously within 4-8 hours. |
|
- |
- |
- |
AMIdoesn’t occur if still suspec,can test continuously within 2-4 hours. |
The Advice for Combined Applicationof Cardiac Marker
1. TheSignificance for Joint Test of Myocardial Infarction Three Item ( cTnI / Myo / CK-MB )
Diagnosis of ACS in the early stage ;
Prognostic assessment and risk stratification for ACS ;
Testing the degree of myocardial damage caused by cardiac surgery ;
Estimating the size for Myocardial infarction ;
AMI thrombolytic and marker of interventional therapy.
2. When testing the acute myocardial infarction,H-FABP is "gold partner" of cTnI.
|
H-FABP |
cTnI |
Significance |
|
+ |
+ |
Myocardial infarction exists. |
|
+ |
- |
May be an early stage of myocardial infarction,need to monitor continuously. |
|
- |
+ |
Myocardial infarction exist,and its time may be more than 6 hours. |
|
- |
- |
Testing again after 1-2 hours,can exclude myocardial infarction if still negative. |
○The Advice for Usage of Myocardial Damage Marker
<2Hours H-FABP+Myo
2-6Hours H-FABP+Myo+cTnI+CK-MB
4-8Hours cTnI+CK-MB
>8Hours cTnI
○Introduction of Product and Clinical Application
○ Emergency Department
Distinguishingand diagnosis foracute expiratory dyspnea.
Early diagnosis for acute myocardial infarction.
○ Pediatrics / NeonatologyDepartment
Auxiliary diagnosis and monitor of asphyxia neonatorum.
Auxiliary diagnosis of viral myocarditis for children.
○ NephrologyDepartment
Monitoring myocardial damage for patient of Chronic renal failure.
Car enal syndrome related to heart failure.
○Department of Cardiology / Internal Medicine
Distinguishingand diagnosis for heart failure,prognosis judgement and guide treatment.
Early diagnosis for acute myocardial infarction,recurrent monitoring,guide treatment.
Auxiliary diagnosis,guide treatment,prognosis evaluation for coronary syndrome.
○ Surgery
Screening for perioperative and postoperative cardiovascular risk.
○The Platform of Test for Cardiac Marker
Central Laboratory
Emergency
ICU
Community Care
Private Clinic

The combination of Item for Clinical Examination
|
Disease |
The Item of Test |
|
Heart Failure |
NT-proBNP |
|
Myocardial Infarction |
H-FABP、cTnI、Myo、CK-MB |
|
Pectoralgia |
NT-proBNP、H-FABP、cTnI、Myo、CK-MB |
|
Expiratory Dyspnea |
D-Dime、NT-proBNP、H-FABP、cTnI、Myo、CK-MB |
|
Pulmonary Vein / Deep Vein Thrombosis |
D-Dimer |
|
Infectious Diseases |
PCT |
Member Information
| Shenzhen Bio Cup BioTech Co.,Ltd. | |
|---|---|
| Country/Region: | Guang Dong - China |
| Business Nature: | Manufacturer |
| Phone: | 68773786 |
| Contact: | Andey Lee (Sales Manager ) |
| Last Online: | 27 May, 2014 |
Related Products of this Company
-
Myo from POCT
US $4
-
CK-MB From POCT
US $3
-
H-FABP
US $8
-
Adding Sample Pipettor
US $60