| Model: | 500、501、502、505 |
|---|---|
| Brand: | NeuCognic |
| Origin: | Made In China |
| Category: | Services / Therapies |
| Label: | CVA or stroke , Multiple Sclerosis , TBI |
| Price: |
-
|
| Min. Order: | - |
Product Description
-Theory of Operation
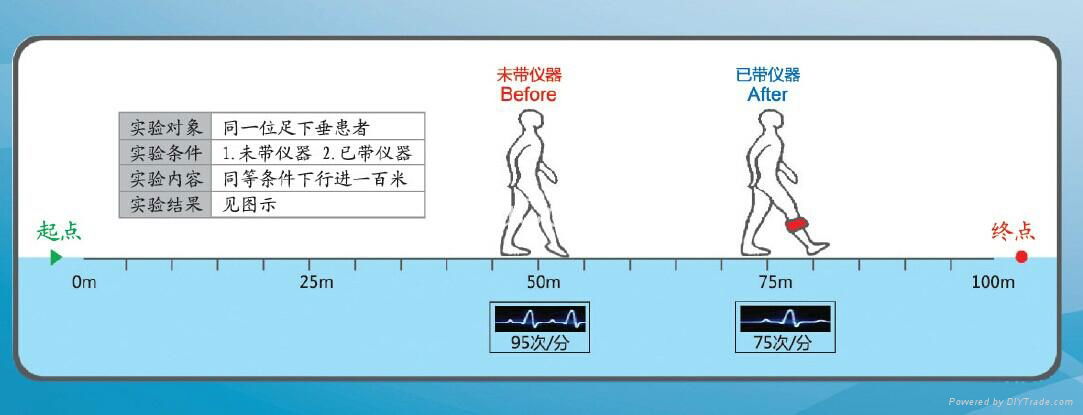
The DC-L-500 is an FES (functional electrical stimulation) device. While walking, the Gait Sensor (tilt or heel-pressure sensor) detects the swing phase of the gait cycle. The stimulator is trigged by the sensor signal to deliver electrical pulses to the common peroneal nerve and the tibialis anterior muscle through the electrodes, thus may improve the gait. (The DC-L-500 is protected by patent)
-Indications for Use
CVA or stroke
Multiple Sclerosis
TBI
Spinal Cord Injury
Foot drop by central nervous system injury
-Product advantages
Humanized design
Handheld Programmer
Subminiature Stimulator
Rechargeable Lithium ion Battery
Proprietary electrode holder
Biphasic wave, good clinical results andlow possibilityto burn theskin
The Patent technology of automatic adaptive gait detection, andhigh intelligence
-Instructions Below Must Be :
Patients with any electrical or metallic implant, including implanted cardiac pacemaker or defibrillator, should not use the DC-L-500.
Patients with epilepsy should not use the DC-L-500.
Prohibit the use of the DC-L-500 during pregnancy.
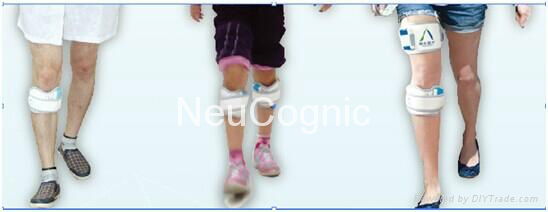
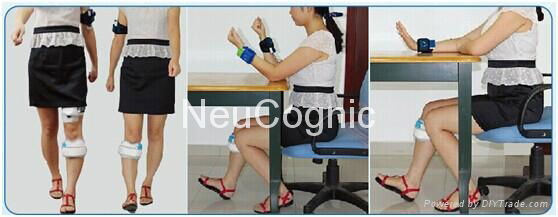
-Fitting the DC-L-500
Disease assessment-electrodepositioning- Parameter Setting
-Using the DC-L-500
Put the cuff on the leg- Turn on the power
Member Information
| Jiangsu Neucognic NeuCognic Medical Co.,Ltd. | |
|---|---|
| Country/Region: | Jiang Su - China |
| Business Nature: | Manufacturer |
| Phone: | 52192159 |
| Contact: | Charles ( marketing manager) |
| Last Online: | 29 Mar, 2016 |