| Model: | INC-052 |
|---|---|
| Brand: | SPAN |
| Origin: | Made In China |
| Category: | Chemicals / Biochemical |
| Label: | strep A rapid test , strep A test , strep A antigen test |
| Price: |
US $30
/ SHEET
|
| Min. Order: | 50 SHEET |
Product Description
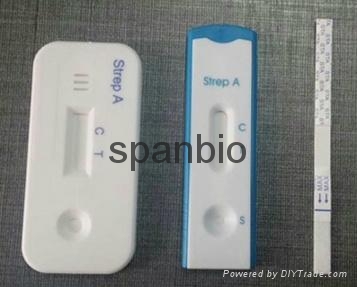
~A rapid test for the qualitative detection of Strep A antigen in throat swab specimens. For professional in vitro diagnostic use only.
INTENDED USE
The Strep A Rapid Test Cassette (Throat Swab) is a rapid chromatographic immunoassay for the qualitative detection of Strep A antigen from throat swab specimens to aid in the diagnosis of Group A Streptococcal infection.
SUMMARY
Streptococcus pyogenes is non-motile gram-positive cocci, which contains the Lancefield group A antigen that can cause serious infections such as pharyngitis, respiratory infection, impetigo, endocarditis, meningitis, puerperal sepsis, and arthritis.1 Left untreated, these infections can lead to serious complications, including rheumatic fever and peritonsillar abscess.2 Traditional identification procedures for Group A Streptococci infection involve the isolation and identification of viable organisms using techniques that require 24 to 48 hours or longer.3,4
The Strep A Rapid Test Cassette (Throat Swab) is a rapid test to qualitatively detect the presence of Strep A antigen in throat swab specimens, providing results within 5 minutes. The test utilizes antibodies specific for whole cell Lancefield Group A Streptococcus to selectively detect Strep A antigen in a throat swab specimen.
PRINCIPLE
The Strep A Rapid Test Cassette (Throat Swab) is a qualitative, lateral flow immunoassay for the detection of Strep A carbohydrate antigen in a throat swab. In this test, antibody specific to Strep A carbohydrate antigen is coated on the test line region of the test. After the test strip is immersed into a specimen, the extracted throat swab specimen reacts with an antibody to Strep A that is coated onto particles. This mixture migrates up the membrane to react with the antibody to Strep A on the membrane and generate a colored line in the test line region. The presence of this colored line in the test line region indicates a positive result, while its absence indicates a negative result. To serve as a procedural control, a colored line will always appear at the control line region, indicating that proper volume of specimen has been added and membrane wicking has occurred.
Materials Provided
Test Cassettes
Strep A Reagent A (2M Sodium Nitrite)
Strep A Reagent B (0.4M Acetic Acid)
Sterile swabs
Workstation
Package insert
Test tubes
Materials Required But Not Provided
Timer
PRECAUTIONS
1. For professional in vitro diagnostic use only. Do not use after expiration date.
2. Do not eat, drink or smoke in the area where the specimens and kits are handled.
3. Handle all specimens as if they contain infectious agents. Observe established precautions against microbiological hazards throughout the procedure and follow the standard procedures for proper disposal of specimens.
4. Wear protective clothing such as laboratory coats, disposable gloves and eye protection when specimens are assayed.
5. Humidity and temperature can adversely affect results.
6. Do not use test if pouch is damaged.
7. Reagent B contains an acidic solution. If the solution contacts the skin or eye, flush with large volumes of water.
8. The positive and negative controls contain sodium azide (NaN3) as a preservative.
9. Do not interchange reagent bottle caps.
10. Do not interchange external control solution bottle caps.
STORAGE AND STABILITY
Store as packaged in the sealed pouch at room temperature or refrigerated (2-30°C). The test strip is stable through the expiration date printed on the sealed pouch. The test strip must remain in the sealed pouch until use. DO NOT FREEZE. Do not use after the expiration date.
SPECIMEN COLLECTION AND PREPARATION
1. Only use reagents and sterile swabs provided in the kit.
2. Collect the throat swab specimen with the sterile swab that is provided in the kit. Swab the posterior pharynx, tonsils and other inflamed areas. Avoid touching the tongue, cheeks and teeth with the swab.5
3. Testing should be performed immediately after the specimens have been collected. Swab specimens may be stored in a clean, dry plastic tube for up to 8 hours at room temperature or 72 hours at 2 8°C. Transport swabs containing modified Stuart’s or Amies medium?can also be used with this product.
4. If a culture is desired, lightly roll the swab tip onto a Group A selective (GAS) blood agar plate before using the swab with the Strep A Rapid Test Cassette (Throat Swab).
DIRECTIONS FOR USE
Allow the test device, specimen, reagents, and/or controls to reach room temperature (15-30°C) prior to testing.
1. Remove the test device from the sealed foil pouch and use it as soon as possible. Best results will be obtained if the test is performed immediately after opening the foil pouch.
2. Hold the Reagent A bottle vertically and add 4 full drops (approximately 240 uL) of Reagent A to an extraction test tube. Reagent A is red in color. Hold the Reagent B bottle vertically and add 4 full drops (approximately 160 uL) of Reagent B. Reagent B is colorless. Mix the solution by gently swirling the extraction test tube. The addition of Reagent B to Reagent A changes the color of the solution from red to yellow.
3. Immediately add the throat swab into the extraction test tube of yellow solution. Agitate the swab by rotating it at least 10 times. Leave the swab in the extraction test tube for 1 minute. Then express the liquid from the swab head by rolling the swab against the inside of the tube and squeezing the tube as the swab is withdrawn. Discard the swab.
4. Fit the dropper tip on top of the extraction test tube. Place the test device on a clean and level surface. Add 3 full drops of solution (approx. 100 μL) to the specimen well (S) and then start the timer.
5. Wait for the colored line(s) to appear. Read the result at 5 minutes. Do not read the result after 10 minutes...
INTERPRETATION OF RESULTS (Please refer to the illustration above)
POSITIVE: Two lines appear. One colored line should be in the control line region (C) and another apparent colored line should be in the test line region (T). A positive result indicates that Strep A antigen is detected in the specimen.
NEGATIVE: One colored line appears in the control line region (C). No line appears in the test line region (T). A negative result indicates that Strep A antigen is not present in the specimen, or is present below the detectable level of the test. The patient’s specimen should be cultured to confirm the absence of Strep A infection. If clinical symptoms are not consistent with results, obtain another specimen for culture.
INVALID: Control band fails to appear. Results from any test which has not produced a control band at the specified reading time must be discarded. Please review the procedure and repeat with a new test. If the problem persists, discontinue using the kit immediately and contact your local distributor.
NOTE: 1. The intensity of the color in the test line region (T) will vary depending on the concentration of Strep A antigen present in the specimen. Therefore, any shade of color in the test line region (T) should be considered positive.
2. Insufficient specimen volume, incorrect operation procedure, or performing expired tests are the most likely reasons for control band failure.
QUALITY CONTROL
Internal Quality Control
Internal procedural controls are included in the test. A colored line appearing in the control line region (C) is an internal positive procedural control. It confirms sufficient specimen volume, adequate membrane wicking and correct procedural technique.
External Quality Control
In addition to your laboratory’s standard quality control procedures, it is recommended that a positive and negative external control be tested at least once within each test kit and by each operator performing testing within a kit. This will verify that the reagents and test strips are working properly and the operator is able to correctly perform the test procedure. External positive and negative controls are supplied in the kit.
Procedure for External Quality Control Testing
1. Add 4 full drops of Reagent A and 4 full drops of Reagent B into an extraction test tube. Mix the solution by gently swirling the extraction tube.
2. Add 1 full drop of positive or negative control solution into the extraction tube, holding the bottle vertically.
3. Place a clean swab into this extraction tube and agitate the swab in the solution by rotating it at least 10 times. Leave the swab in the extraction tube for 1 minute. Then express the liquid from the swab head by rolling the swab against the inside of the extraction tube and squeezing the extraction tube as the swab is withdrawn. Discard the swab.
4. Continue with Step 4 of Directions For Use.If the controls do not yield the expected results, do not use the test results. Repeat the test or contact your distributor.
| Payment Terms: | T/T in advance WESTERN UNION |
|---|---|
Member Information
| Span Biotech Ltd | |
|---|---|
| Country/Region: | Guang Dong - China |
| Business Nature: | Manufacturer |
| Phone: | +86-13417551798 |
| Contact: | Anna Lee (Int'l Mkt. Executive) |
| Last Online: | 07 Nov, 2017 |
Related Products of this Company
-
One Step Influenza A Test
US $3
-
Giardia Ag Feces Rapid Test uncut sheet
US $85
-
Canine Distemper Virus Ag Rapid Test
US $3
-
Feline Leukemia Virus Ag Rapid Test
US $3
-
Bovine Brucella Ab Test
US $3
-
Dengue NS1 antigen rapid test uncut
US $50
-
Giardia Ag Feces Rapid Test uncut sheet
US $85
-
Canine Parvovirus Ag Test
US $3
-
Dengue IgG/IgM rapid test uncut sheet
US $40
-
Foot and Mouth Disease NSP Ab Test
US $3