| Model: | MK50DL |
|---|---|
| Brand: | Makcom |
| Origin: | Made In China |
| Category: | Home Supplies / Personal Care Appliance |
| Label: | Fingertip Oximeter , Pulse Oximeter , Oximetry |
| Price: |
US $30
/ pc
|
| Min. Order: | 1 pc |
Product Description
Major Features
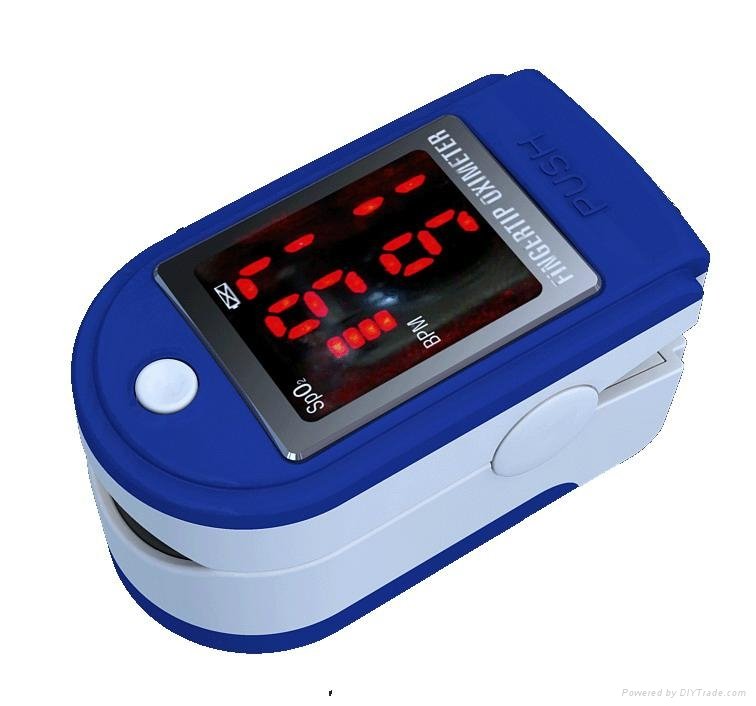
Integrated with SpO2 probe and processing display module
Small in volume,light in weight and convenient in carrying
Operation of the product is simple ,low power consumption
SpO2 value display
Pulse rate value display, bar graph display
Low-voltage indication: low-voltage indicator appears before working abnormally which is due to low-voltage
Automatically power off function: when the device is under the state of measuring interface . it will automatically power off within 5 seconds if the finger falls out of probe
Various color of cover can be selected
Main performance
Display Mode: LED display
SpO2 Measuring Range: 0%~100%, (the resolution is 1%).
Accuracy: 70%~100%: ±2% ,Below 70% unspecified.
PR Measuring Range: 30bpm~250bpm, (the resolution is 1bpm)
Accuracy: ±2bpm or ±2% (select larger)
Measurement Performance in Weak Filling Condition:
SpO2 and pulse rate can be shown correctly when pulse-filling ratio is 0.4%. SpO2 error is ±4%, pulse rate error is ±2 bpm or ±2% (select larger).
Resistance to Surrounding Light: The deviation between the value measured in the condition of man-made light or indoor natural light and that of darkroom is less than ±1%.
Power Consumption: less than 25 mA
Voltage: DC 3.0V
Power Supply: 1.5V (AAA size) alkaline batteries × 2
Battery Working Hour: The minimum continually work time is 24 hours, theoretical number is 56 hours.
Safety Type: Interior Battery, BF Type
Content:
1. Pulse oximeter
2. Carry case
3. Landyard
4. Manual
Member Information
| Makcom Medical Electronics Co ,.ltd | |
|---|---|
| Country/Region: | He Bei - China |
| Business Nature: | Manufacturer |
| Phone: | 13303354359 |
| Contact: | Serena (Sales Manager ) |
| Last Online: | 06 Feb, 2013 |