| Model: | MK50D |
|---|---|
| Brand: | Makcom |
| Origin: | Made In China |
| Category: | Home Supplies / Personal Care Appliance |
| Label: | Pulse Oximeter , Oximetry , Fingertip Pulse Oxim |
| Price: |
-
|
| Min. Order: | 1 pc |
Product Description
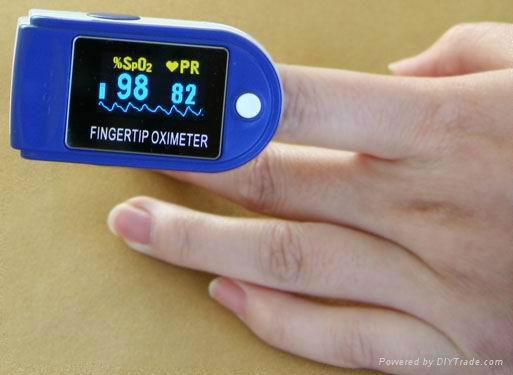
CONTEC Brand CE& FDA Approved Fingertip Pulse Oximeter Spo2 Monitor CMS 50D

Major Features
1.Integrated with SpO2 probe and processing display module
2.Small in volume,light in weight and convenient in carrying
3.Operation of the product is simple, low power consumption
4.SpO2 value display
5.Pulse rate value display, bar graph display
6.Pulse waveform display
7.The display mode can be changed
8.Screen brightness can be changed
9.Low-voltage indication: low-voltage indicator appears before working abnormally which is due to low-voltage
10.Automatically power off function: when the device is under the state of measuring interface . it will automatically power off within 5 seconds if the finger falls out of probe
11.Display format can be saved after power off
Main performance
1. Display Mode:0.96" Dual-color OLED display(blue and yellow)
2. Screen Resolution:128*64
3. SpO2 Measuring Range:0%~100%, (the resolution is 1%).
4.Accuracy:70%~100%:±2% ,Below 70% unspecified.
5.PR Measuring Range:30bpm~250bpm, (the resolution is 1bpm)
6. Accuracy:±2bpm or ±2% (select larger)
7. Measurement Performance in Weak Filling Condition:SpO2 and pulse rate can be shown correctly when pulse-filling ratio is 0.4%. SpO2 error is ±4%, pulse rate error is ±2 bpm or ±2% (select larger).
8. Resistance to surrounding light: The deviation between the value measured in the condition of man-made light or indoor natural light and that of darkroom is less than ±1%
9. Power Consumption:less than 30mA
10. Voltage: DC 2.6V~3.6V
11.Power Supply:1.5V (AAA size) alkaline batteries × 2
12. Battery working hour: Theoretical number is 32 hours.
13. Safety Type:Interior Battery,BF Type
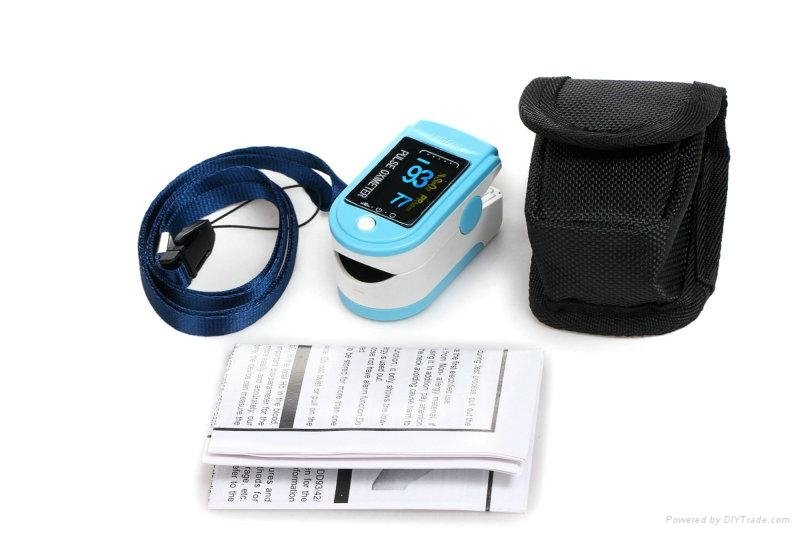
Accessories Sell in standard
1. a hanging rope
2. a user manual
Physical Identity
1.Dimension:57(L) × 31(W) × 32(H) mm
2.Weight:About 50g (with the batteries)
Warranty
3 years and whole life free repair
Certification
CE & FDA



Pictures of other model for your kind reference :

FDA K Number
CMS50D/L/DL Pulse Oximeter: K082641

ABOUT US :CONTEC Medical Systems focusing on research, manufacture and distribution of medical equipments, was founded in 1992 as a professional medical equipments producer. Our Products include followings :
ECG / ECG holter EEG / EEG Holter B-ultrasound Scanner
Patient Monitor Fetal doppler Fetal Monitor
Spo2 Monitor Stethoscope Medical Image

Member Information
| Makcom Medical Electronics Co ,.ltd | |
|---|---|
| Country/Region: | He Bei - China |
| Business Nature: | Manufacturer |
| Phone: | 13303354359 |
| Contact: | Serena (Sales Manager ) |
| Last Online: | 06 Feb, 2013 |